Understanding Plant Nutrition: Limestone, Calcium And Magnesium
The reason for adding limestone to a container media, besides pH control, is to supply calcium and, depending on the limestone, magnesium. In this article, we will discuss the nutrient content of different types of limestone and how limestone influences calcium and magnesium nutrition.
Limestone Nutrient Content
There are four types of carbonate-based limestone that are available. Calcite is pure calcium carbonate (CaCO3, 40 percent Ca). Calcitic lime is composed of mostly CaCO3 (> 30 percent Ca) with some MgCO3 (<5 percent Mg). Dolomitic lime contains less CaCO3 (<30 percent Ca) and more MgCO3 (>5 percent) than calcitic lime. Finally, dolomite also contains CaCO3 and MgCO3, but at a specific ratio of 22 percent Ca to 13 percent Mg.
Calcium hydroxide (Ca(OH)2), also known as hydrated lime, or calcium magnesium hydroxide (CaMg(OH)4), also known as dolomitic hydrated lime, are sometimes used as a fast reacting liming source because they will react quickly and leave no residual. Calcium hydroxide contains approximately 54 percent Ca, while calcium magnesium hydroxide contains about 30 percent Ca and 18 percent Mg.
Sometimes the calcium and magnesium content of the limestone is listed as CaO or MgO on the label. To convert CaO to actual Ca, multiply the CaO value by 0.71. To convert MgO to actual Mg, multiply the MgO value by 0.6.
Reactive Lime And Calcium
Reactive lime is that fraction of the incorporated limestone that causes the media pH to increase initially after mixing. When limestone is incorporated into a media, the important reactions that occur are:
| 1 | 2peat-H <—> 2peat- + 2H+ |
| 2 | CaCO3 + 2H+ <—> Ca2+ + H2O(water) + CO2 (gas) |
| 3 | Ca2+ + 2peat- <—> Ca-peat2 |
After the peat and lime are done reacting and the equilibrium pH is reached, the peat is less acidic and contains calcium on its exchange sites. When limestone is suggested as a source of calcium for the plant, it is usually the exchangeable calcium left over from the reactive lime fraction.
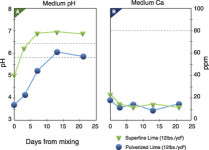
Research at Michigan State University tested the effect that a dolomitic limestone had on pH and calcium concentrations for the first four weeks after mixing (Figure 1). It was shown that the reactive fraction of the limestone had little effect on root-medium calcium concentrations, even as the limestone continued to react.
It appears that once the calcium from the reactive limestone is bound to the peat, it is not released back into the soil solution under most conditions. Since nutrients must be soluble to be taken up by the plant, the bound calcium does not influence calcium nutrition, either in the short term (mixing to stable pH) or the long term (stable pH till the end of the crop). It also may explain the reason that cation exchange capacity of a limed peat has minimal influence on pH buffering.
Residual Lime And Calcium
Just like with pH buffering, it’s the residual lime fraction that influences calcium buffering. Figures 2 and 3 show the results from an experiment at Michigan State University in which the same peat/perlite media was prepared using two different types of lime. The low-residual treatment contained a hydrated lime, which reacted quickly and completely, leaving no residual. The high-residual lime treatment contained a dolomitic lime with a large residual fraction. Impatiens were grown for 17 weeks in both media using one of three fertilizer solutions.
The acidic nutrient solution (NS) was composed of water with 20 ppm alkalinity and a fertilizer containing 50 percent NH4-N:50 percent NO3-N. The neutral NS was composed of water with 120 ppm alkalinity and a fertilizer containing 25 percent NH4-N:75 percent NO3-N. The basic NS was composed of water with 120 ppm alkalinity and a fertilizer with 3 percent NH4-N:97 percent NO3-N. The calcium concentrations also differed between the fertilizer solutions with the acidic NS containing 15 ppm Ca, the neutral NS containing 50 ppm Ca and the basic NS containing 150 ppm Ca.
When the acidic NS was used, both media and shoot-tissue calcium concentrations were significantly higher in plants grown in media containing the high residual lime fraction compared to those grown in the media without the residual lime (Figures 2 and 3).
However, when less acidic fertilizer solutions were used, there was little difference in the media or shoot-tissue calcium concentrations of plants grown in the two media.
These results suggest that the residual lime contained in the medium after the equilibrium pH was reached did not, in itself, buffer the root-medium Ca concentrations. Instead, the increase in root-medium and shoot-tissue Ca resulted from the reaction of the acidic NS with the residual lime. Decreasing the acidity of the fertilizer solution, either by reducing the amount of NH4-N in the fertilizer or by increasing the alkalinity of the water, almost completely negated the residual lime as a calcium source.
Magnesium
The effect that limestone has with magnesium nutrition follows the same pattern as with calcium.
The reactive lime fraction did not influence magnesium concentrations in the media, even as the limestone continued to react. While the residual lime fraction was a source of magnesium under acidic conditions, reducing the acidity of the fertilizer solution negated the residual lime as a magnesium source.
The chemistry of the limestone will also influence magnesium nutrition. The research presented in this article used a dolomitic lime. Other lime sources can contain less magnesium (and more calcium). It would be expected that using a limestone with a lower percentage of magnesium would further reduce the limestone as a magnesium source.
Limestone can be an important source of calcium and magnesium for a crop, but it may not be a consistent source because it is not available under all conditions. In addition to limestone, starter fertilizers are often incorporated into media in order to get consistent concentration of both macronutrients and micronutrients at time of planting. In next month’s article, we will discuss sources for starter fertilizers and their persistence in the media over time.









