Understanding Plant Nutrition: Limestone and pH
In last month’s article, we stated that cation exchange capacity (CEC) does not play an important role in pH, calcium and magnesium buffering. The question should then be asked: Is growing in a soilless media similar to hydroponics when it comes to pH or nutritional management?
Growing in a soilless root medium is not the same as growing with hydroponics because there are sources of buffering found in container media. One of the strongest buffers for long-term pH and nutritional management is limestone. In this article, we will focus on the effect limestone has on pH management by discussing why limestone needs to be added to a soilless media, and the difference between reactive and residual limestone.
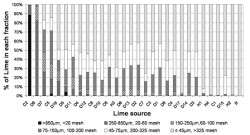
Dry sieving method was used for carbonate liming samples (calcitic: C1-8, dolomitic:
D1-17 and reagent grade CaCO3: R), whereas wet sieving method for
hydrated materials (H1-4). Research by Paul Fisher, Jinsheng Huang and Bill Argo.
Requirement
There are three Rs of limestone incorporation —requirement, reactivity and residual. The lime requirement is the amount of acidity contained in a given volume of media or media component that has to be neutralized to raise the pH to some desired level, usually around 6.0. This acidity comes from the acidic hydrogen ions contained on the exchange sites. In other words, CEC does not affect buffering, but it does affect lime requirements.
While peat tends to be the most acidic component used to produce soilless media, it is not the only component that contains acidic hydrogen ions. Any unamended organic material, like bark, coir or compost, can have enough acidic hydrogen ions on its exchange sites to require the addition of limestone to increase the media pH. Some secondary media components, like perlite or expanded polystyrene, are inert and do not directly affect lime rates. Other components, like expanded vermiculite or rockwool, can have an extremely high pH and can act as a liming agent.
Bulk density also influences lime requirement because it affects the amount of material (and amount of acidity) contained in a given volume. For example, the more degraded the peat, the higher the lime requirement. In most cases, it is the finer particle size (higher bulk density) of the more degraded peat that causes most of the higher lime requirement when compared to coarser (lower bulk density), less degraded peats. This means that anything done to a media component like peat that breaks down structure (decreases particle size and increases bulk density) can increase the lime requirement.
Many people will focus on the initial unamended pH of a specific media component when trying to determine lime requirements. In fact, media pH only gives you an indication of the concentration of acid dissolved in water, not the total amount of acid contained in the media that needs to be neutralized. So, just like water pH tells you nothing about the alkalinity concentration in that water, initial pH of an unamended media or media component tells you nothing about its lime requirement.
Reactivity
Limestone reactivity describes both the amount of pH increase that can be expected from a given incorporation rate and the time needed for the media pH to stabilize (usually 7 to 14 days after mixing at greenhouse temperatures and adequate moisture condition). For carbonate-based limestones, the reactivity is affected by a number of factors including the chemistry of the limestone [calcitic lime (CaCO3) versus dolomitic lime (CaMg(CO3)2)], acid neutralizing value (purity), surface area (hardness) and the particle size distribution.
Research at the University of New Hampshire and the University of Florida demonstrated that the particle size distribution best described the reactivity of a limestone. The limestone particle size that is responsible for causing the media pH to increase initially is that fraction which is finer than 150 µm (will pass a 100 mesh screen).
In general, anything finer than 75 µm (will pass a 200 mesh screen) will react completely and rapidly, reaching the equilibrium pH within a few days to a week. A lime particle between 75 and 150 µm (100 to 200 mesh) will still react almost completely, but rate of the reaction will be slower. The particle size distribution for 29 limes used in commercial production and analytical grade CaCO3 is presented in Figure 1.
On any bag of lime, the particle size distribution for that limestone should be specified. For example, a pulverized limestone will have minimum of 65 percent passing a 150 µm (100 mesh) screen, a superfine limestone will have minimum of 65 percent passing a 75 µm (200 mesh) screen, while a microfine limestone will have minimum of 95 percent passing a 45 µm (325 mesh) screen.
However, with the exception of the microfine limestones, these definitions can be misleading. For example, the lime designated as D11 in Figure 1 is sold as a pulverized dolomitic lime. With this lime, 79 percent of the material passes a 150 µm (100 mesh) screen, while 58 percent of the material passes a 75 µm (200 mesh) screen, making the D11 lime finer and more reactive than its designation suggests.
Residual Lime
Not all the limestone incorporated into a root medium initially reacts to bring the pH up. The limestone that remains unreacted in the media after the equilibrium pH is reached is termed residual lime. It is the residual lime that can give significant pH buffering to container media.
Figure 2 shows the results from an experiment at Michigan State University, in which the same peat/perlite media was prepared using two different types of lime. The low-residual treatment contained a hydrated lime, that reacted quickly and completely leaving no residual. With this treatment, the only buffering comes from the CEC of the peat. The high-residual lime treatment contained a dolomitic lime with a large residual fraction. Impatiens were grown with an acidic fertilizer solution for 17 weeks in both media.
The medium-pH changed much more in the medium containing hydrated lime, because there was no residual lime in that medium to buffer the pH over time. In this experiment, it is important to note that the peat, the plant species and the volume of acidic fertilizer solution were similar for both treatments. The only difference was in the lime used.
The particle size that affects residual lime is between 850 and 150 µm (20 to 100 mesh) (Figure 1). When these coarse particles are incorporated into a medium, some of the lime reacts to help increase the media pH, but some also remains as residual lime for buffering. With the 850 to 250µm (20 to 60 mesh) fraction, about 65 percent of the lime reacts and 35 percent will remain as residual lime. That means if 10 pounds of this lime fraction were incorporated into a container medium, 6.5 pounds would react to bring the pH up, while the remaining 3.5 pounds would remain as residual lime.
In comparison, only about 10 percent of the 250 to 150 µm (60 to 100 mesh) fraction will remain as residual lime and with the finer fractions being almost completely reactive.
Sometimes growers will use very coarse lime (>850 µm or coarser than a 20 mesh) in their media to give buffering to long-term crops. The idea is that these very large particles will help keep the pH up by dissolving very slowly. Research has shown that these very coarse fractions are almost completely unreactive and have no effect on either short-term or long-term pH management.
Limestone can have a significant effect on pH management. It is used to increase the media pH to an acceptable level for growth, and can affect long-term pH buffering. The other reason that limestone is added to a media is to supply calcium and magnesium to the crop. In next month’s article, we will discuss how limestone affects calcium and magnesium nutrition.