Fertilizer Changes Growing Mix pH

Use of acid forming fertilizers on geraniums can drive the pH down, resulting in trace element toxicity.
Compared to pH management, fertility management seems easy: you either fertilize or you don’t. Commercial water soluble fertilizers turn the water a reassuring blue color. With controlled release fertilizers, you can actually see and squeeze the prills (firm prills contain fertilizer while soft ones are empty). Growing mix pH, however, cannot be visually judged, even by the crudest methods, until plant growth is impacted.
No grower wants to be alerted to pH problems by plant symptoms. Media mixes must be tested to monitor pH. For proper pH management, it is important to understand the four factors influencing pH. These are the mix, water, fertilizer and crop.
Growing Mix pH Levels Change As Elements Are Introduced
The pH is just a measure of the number of hydrogen ions in the mix, measured on a scale of 1 to 14. If there are lots of hydrogen ions, the pH will be low and the mix will be acidic. Few hydrogen ions result in a higher, more basic pH. When the pH is 7, the mix is neutral — neither acidic nor basic. Mix pH is important because it impacts the availability of the various ions of fertilizer nutrients.
When making a growing mix, limestone must be added to adjust the starting pH to a desired point — typically 6.0. Usually, the lime used is crushed dolomite, a mixture of calcium and magnesium carbonate. When the mix is first watered, the dolomite starts to dissolve. The carbonate fraction of the dolomite reacts with the water, tying up hydrogen ions and reducing their number, resulting in higher pH. The calcium and magnesium contained in the lime do not tie up hydrogen ions but do compete for exchange sites in the mix. They do not directly influence the pH.
This initial pH adjustment consumes some of the dolomite. At pH 6.0, there are some, but not a lot, of hydrogen ions in the mix. Because there are few hydrogen ions to tie up, the remaining dolomite is held in reserve.
Even though the pH was adjusted when the mix was made, growing mixes have only a limited ability to resist pH changes during crop production. The three factors that can move the pH away from the starting point are the type of fertilizer used, the properties of the irrigation water and the crop being grown.
Fertilizer Classifications Are Important In Managing pH
Fertilizers can be classified by their effects on growing mix pH. Acidic or acid-forming fertilizers lower mix pH. Basic fertilizers can cause the mix pH to increase. A third class, neutral or non-acid forming fertilizers, have no effect on pH.
Fertilizers can be mixtures of many chemical compounds. With most commonly used greenhouse fertilizers, nitrogen sources are the primary causes of acidity or basicity. A 20-20-20 mix, for example, is a mixture of potassium nitrate, ammonium phosphate and urea. Each of the three ingredients contains a different nitrogen source. The three sources used are nitrate nitrogen, ammonium nitrogen and urea nitrogen. Nitrate nitrogen has a basic affect, raising pH, while ammonium and urea nitrogen are acid forming, lowering pH.
A fertilizer’s effect on pH depends on the ratio of nitrate nitrogen to ammonium and/or urea nitrogen. The more nitrate, the more basic and the more ammonium and/or urea, the more acidic. This information is prominently shown on the fertilizer label. The material’s percentage of nitrogen will be broken out into percentages of nitrate, ammonium and/or urea. For any given fertilizer, these percentages can be used to predict its effect on mix pH.
With soluble fertilizers, it is easy to determine the potential effect on mix pH. Each bag label shows a statement of potential acidity or basicity in terms of pounds of limestone (calcium carbonate) per ton of fertilizer.
For acid-forming fertilizers, the statement indicates the poundage of limestone required to neutralize the acidity produced by one ton of that fertilizer. For basic fertilizers, the poundage of limestone needed to equal the acid neutralizing power of one ton of the fertilizer is indicated. The poundage numbers have no direct meaning, other than for comparing one fertilizer to another.
Table 1 lists some common soluble fertilizers, along with the potential acidity or potential basicity (from most acid to most basic):
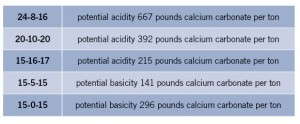
Table 1: Common Soluble Fertilizers
Here are the soluble fertilizers most commonly used in floriculture, including the potential acidity or potential basicity from most acid to most basic.
By examining and comparing statements, a fertilizer’s potential to change mix pH can be judged. The greater the potential acidity number, the more acid-forming the fertilizer. Conversely, the greater the potential basicity number, the more basic the fertilizer.
Controlled release fertilizer (CRF) labels do not show potential acidity/basicity statements. However, most of the commonly used coated, greenhouse-type CRFs contain both nitrate and ammonium in a ratio of about 1:1, making them acidifying. Those that contain urea are somewhat more acid-forming. Higher application rates generate more acidity, and soil incorporation will affect pH faster and to a greater extent than top-dressing. The acidic effects of CRF are much weaker than those of an acidic soluble fertilizer.
Be Aware Of Potential pH Changes When Using A New Fertilizer
In a new situation, predicting the degree to which the mix pH might change can be complex. Type of fertilizer, concentration, frequency of application, degree of leaching and time all influence a fertilizer’s effect on mix pH.
Also of huge importance is the effect of the irrigation water’s alkalinity. Even the plant species being grown influences the growing mix pH. For example, geraniums are acid-producing plants. Just consider the pH management problems experienced by geranium stock plant growers.
Growing mix pH is not a constant. Even though the pH is set when the mix is made, the mix has a limited ability to resist a pH change during crop production.
After the initial watering-in, the mix pH is subject to change by the fertilizer used. Statements made on the fertilizer bags indicate the degree that the material will impact mix pH.
Mix pH management is easier with the understanding that mix pH is modified by a number of known factors, other than the amount of lime in the mix.