How to Address Insoluble Iron Issues in the Greenhouse

Figure 1. Rust-colored stains caused by iron precipitates on the surface of disk filters.
Insoluble iron in water sources causes clogging of irrigation systems and stains on plants when irrigated with overhead irrigation. Iron in water is present in a dissolved form, also known as soluble iron (ferrous state), or in an insoluble form (ferric state).
Dissolved iron is most abundant when the water pH is less than 5.0 and under anoxic (abnormally low amount of oxygen) conditions. When the oxygen in the solution increases, the iron oxidizes and transforms into an insoluble form of iron or iron precipitates.
Dissolved iron also precipitates into insoluble iron when the solution has a basic pH. Iron precipitates, which look like rust particles, stain leaves and accumulate on surfaces of the irrigation systems (Figure 1).
Iron in ground water comes from weathering of minerals, so its presence and concentration vary by location. Deep wells are the only water source prone to the high iron concentration, but not all deep wells have high iron concentration. Water in deep wells remains stagnant and anoxic, keeping the iron in solution. When the water is pumped out of the well, the iron is exposed to oxygen. Then the iron precipitates and accumulates on the irrigation systems. Iron-oxidizing bacteria can also transform iron from soluble to insoluble forms.
Testing Iron in Water Samples
Some growers have reported a reddish-rust color on surfaces in the irrigation system or growing space, and when they send water samples for testing, the results show levels as low as 0.1 ppm iron. These results may be explained by a combination of factors: (1) most horticultural laboratories test for soluble iron because it is the form plants take up; (2) the pH of the sample is around neutral or basic; or (3) the iron precipitated before or after sampling and was inadvertently excluded from the analysis.
Growers who suspect their deep wells have high iron should:
- Measure total iron. Communicate with the laboratory before sending the samples. Ask them about options to test total iron. The laboratories may opt not to filter the sample and lower the pH of the solution to less than 2.0 to transform all the iron into a soluble form (a form the labs typically measure), or they might conduct an alternative test that directly measures total iron.
- Sample properly. Collect the sample in a clean container and at different points in the irrigation systems (closest point to the source and at the emitter). Avoid long storage times and light exposure. Ship samples as soon as possible using airtight containers.
- Interpret the results. The ideal iron level in water sources for crop production is less than 1 ppm soluble iron. There is not a defined threshold of iron in water for clogging (as it depends on the interaction with the factors mentioned above). The test can be used to confirm the visual rust and will help define treatment needs.
Take-Away Lessons for Treatment
Here are a few lessons we have learned from our research and projects.
A grower in Connecticut reported lowering the water pH immediately after withdrawal from the well — to prevent clogging of disk filters throughout the irrigation system — and then readjusting the pH to meet the plant needs immediately before injecting fertilizer and irrigating the crops. This management strategy may solve clogging issues. However, acids are corrosive, and continually injecting them at high concentrations may ultimately damage equipment. There is also a risk of crop damage, if the pH is not properly adjusted at the greenhouse. Hence, close monitoring of the crops is essential.
Oxidation followed by filtration is a simple, relatively inexpensive option to remove iron. Potassium permanganate is a common option to oxidize iron. We evaluated the efficacy of removing 4 ppm iron using potassium permanganate at an acid and basic pH (Figure 2). We observed that if we raised the pH up to 7.5 and then filter, iron was removed by more than 90% with or without potassium permanganate with a five- to 30-minute contact time. Whereas, at a pH of 5.1, potassium permanganate also removed more than 90% of the iron (Figure 2). Potassium permanganate is effective at oxidizing iron in a broad pH range.
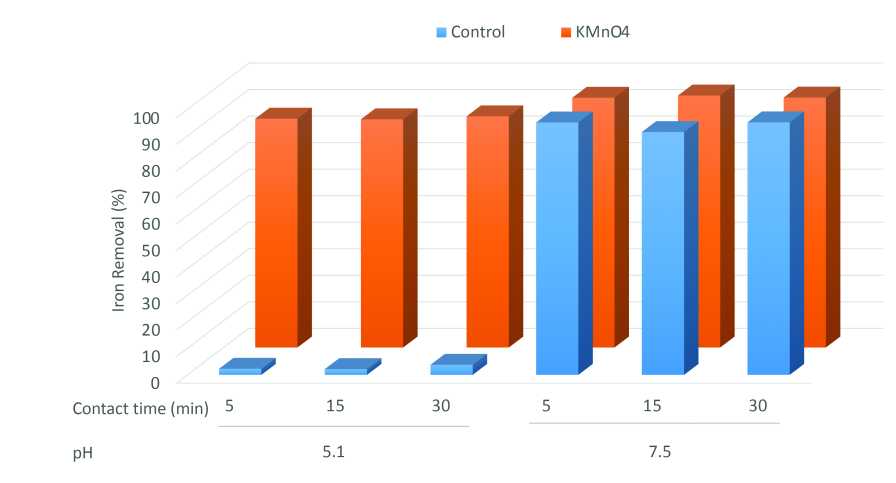
Figure 2. Iron removal after treatment with potassium permanganate (KMnO4) after 5, 15, and 30 minutes contact time. The initial concentration was 4 ppm iron, and the potassium permanganate rate was 1:1 (iron: KMnO4).
A grower in Connecticut reported clogging of the emitters and filters in their operation. Laboratory results reported 0.1 ppm iron. We measured iron in our lab, and the result was 2.5 ppm total iron. While these levels were on the low end, iron oxidation was evident. We also analyzed the microbiome of their water samples and identified that iron-oxidizing bacteria of the genera Acidiferrobacter, Ferriphaselus, Gallionella, Sideroxidans, and TRA3-20 represented 46% of the population in the sample from their well and 22% in the sample at the emitter (Figure 3). In this specific scenario, the grower might benefit by testing an oxidizer (e.g., chlorine and chlorine dioxide) that also control microbes.
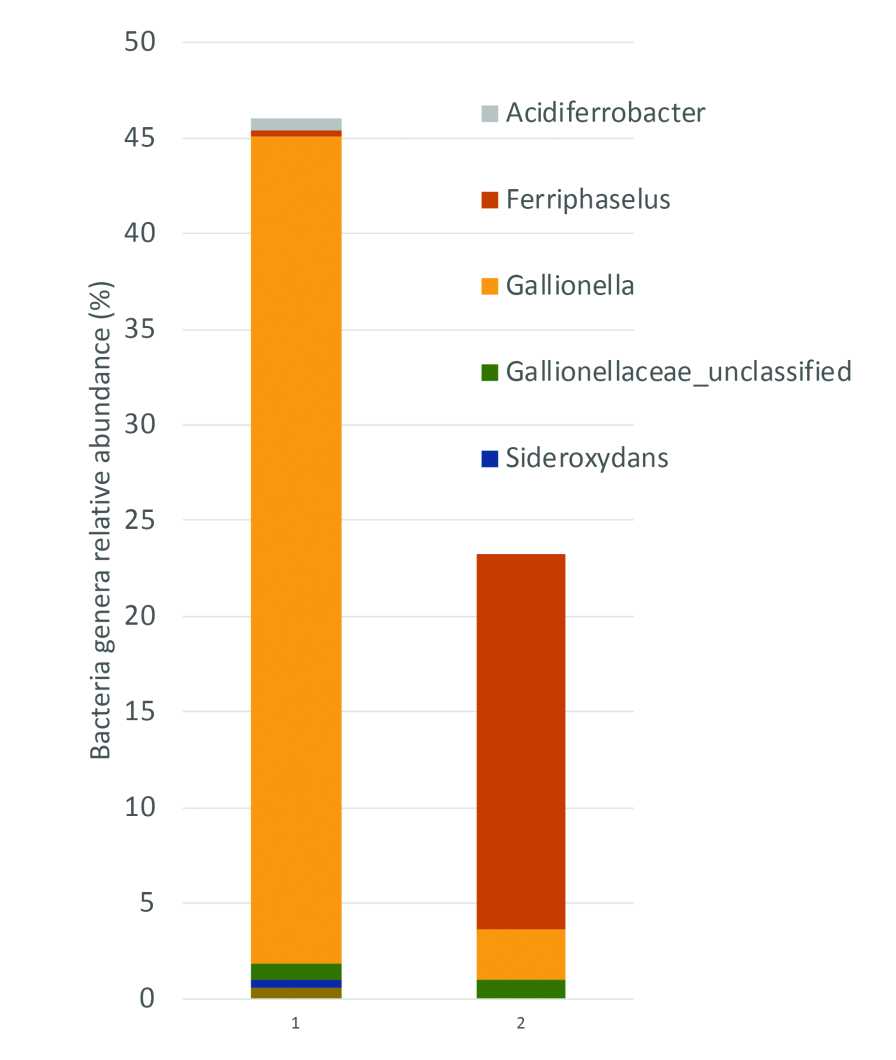
Figure 3. The graph above shows the relative abundance of bacteria that oxidize iron. Samples were from a grower that uses well water and reported clogging of filters and emitters. The rest of the bacteria genera (not shown) had no iron oxidation potential or were unclassified.
Understanding iron forms and factors that affect testing and clogging is only the first step. Iron can be removed from water by oxidation followed by filtration, reverse osmosis, or exchange resins. The ideal technology for each operation will depend on technical factors and business compatibility. More information is available at Cleanwater.org.
This work is supported in part by New England Floriculture Inc.









