5 Tips to Improve Indoor Propagation of Cuttings and Tissue Culture Plants
In some situations, indoor propagation can increase consistency and reduce crop losses (shrinkage) compared with greenhouse production. Shrinkage of 25% or more may occur seasonally for high-valued tissue culture plants such as blueberries or perennial unrooted cuttings (URCs) such as campanula, dicentra, and lavender in a hot greenhouse with excess mist in the summer. It is realistic to half this level of shrinkage with indoor propagation based on research and commercial experience.
Through funding and collaboration from industry partners in the Floriculture Research Alliance, we are running prototype research trials and collecting data from commercial operations. In this article, we provide five tips to help you evaluate this technology based on what we have learned so far.
Choose the Right Crops
Indoor propagation is capital and labor-intensive per tray per week. Choose plants that are high-valued, slow to root, have high shrinkage in the greenhouse, are small (large cell count per tray), and can be started pest- and disease-free (or those pests will multiply in the clean indoor space). This includes many tissue culture plants, and crops such as perennials that are often propagated during the summer when greenhouse environmental control is challenging.
Costs can be reduced and throughput increased by starting cuttings indoors, and after three to four weeks, hardening off transplants in the greenhouse.

Marc Verdel, Director of Horticulture at Battlefield Farms, checks tissue culture liners in the commercial indoor propagation room.
Photo courtesy of University of Florida
Choose the Right Light
Environmental conditions during propagation are hard on most electrical equipment. However, growers are finding that high-quality horticulture fixtures have excellent durability under these high-moisture conditions over several years.
Because electrical use from lights is the main operating cost, choose fixtures with a high photon flux efficacy (PPE, meaning the PAR photon output in µmol·s–1 divided by the electrical input power in watts, or W). Visible light output (lux, foot candles, lumens) is not relevant. A PPE of at least 2 µmol·J–1 is desirable. If your lighting supplier cannot provide this information, look for a different provider. Do-it-yourself lighting with holiday lights or cheap fixtures will not provide adequate intensity, PPE, spectrum, or useful life.
Typical target intensity is 70 to 100 µmol.m-2.s-1 of photosynthetically active radiation (PAR) for initial rooting of cuttings and tissue culture plants, and two to three times that intensity for hardening off, or for growing seedlings. Choose a dimmable fixture so you can increase the light level without needing to move plants.
Run lights for 20 to 24 hours a day to increase the daily light integral, and target at least 5 moles.m-2.day-1 for initial rooting, and 10 or more moles.m-2.day-1 for hardening off. You can use the Daily Light Integral tool in BackPocketGrower.org to make this calculation.
Most growers root successfully with a combination of white, red, and blue LEDs in the 400 to 700 nm range, but research is likely to identify more targeted light spectra in the future.
Control Humidity
High relative humidity is needed during early acclimation of URCs and tissue culture plants. The less you are able to maintain high humidity, the more irrigation is required. Misting increases vertical space between shelves (space-inefficient), and hand watering increases labor.
Reduce airflow to the minimum needed to provide uniform temperature and CO2. This avoids breaking the moist boundary layer around leaves.
For humidifying the entire room and especially in large-scale installations, a fog system is required. On a small scale, a dome can be used (similar in principle to tenting of low-mist crops in a greenhouse).
The light fixtures generate enough heat (target 73˚F to 77˚F for air, leaf, and substrate in most species) so that you only ever need to cool an indoor propagation room. Air-conditioning (AC) also removes humidity, so the humidification and AC systems unfortunately can be fighting each other. Based on our findings, a ballpark figure for electrical cost is that LED lighting will require 80% of total energy use, and 20% in AC.

Good candidates for indoor propagation are small-sized, high-valued crops that have high shrinkage in the greenhouse, such as tissue culture blueberry plants.
Photo courtesy of University of Florida
Transition Crops
Cuttings go through distinct phases of hydration, callus, rooting, and hardening off. A major advantage of indoor propagation is that weather variations are eliminated; however, variable quality among batches of cuttings remains a challenge. Here is an approximate guideline to programming through the phases:
Hydration. The goal is to recover from shipping and transplant stress. Provide close to 100% relative humidity, daily hand watering or misting, and around 70 to 100 µmol.m-2.s-1. Maintain substrate at moisture level 4 (high) in the 1 to 5 scale (see BackPocketGrower.org under “Training” and “Irrigation”). Minimize air movement to avoid wilting.
Callus. The goal is to form callus and root initials. Maintain climate conditions and reduce mist or irrigation so long as cuttings remain turgid.
Root growth. The goal is to produce enough roots to hold the root ball together (have a pullable plug). Maintain climate conditions, but try to reduce substrate moisture level over time to levels 3 to 4. We provide daily foliar nutrient sprays to vigorous species at 150 ppm N from 17-4-17 unless they are sensitive to foliar salts, to reduce nutrient deficiency symptoms. We also provide 800 ppm carbon dioxide to promote growth (from compressed gas).
Hardening off. The goal is to produce a transplant that will survive transport and have success after transplanting. Reduce relative humidity to around 70% to increase nutrient uptake. Increase light level to 150 to 200 µmol.m-2.s-1 or move to a shaded greenhouse for five to seven days before transitioning to full sunlight. We fertigate with each watering with 150 ppm N from 17-4-17 and cycle from 2 to 4 in substrate moisture.
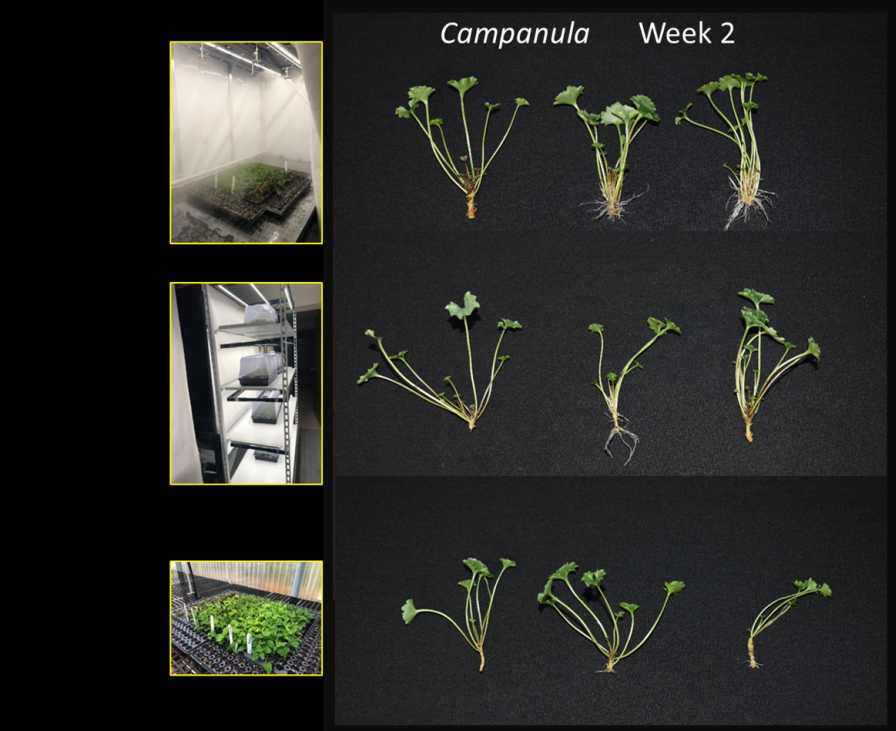
For research at the University of Florida, an indoor fog and mist compartment has LED fixtures, fog injection, mist nozzles, and CO2 gas injection. We also mount LEDs to a shipping light cart and dome system and compare with mist propagation in a greenhouse. This photo shows rooting of Campanula portenschlagiana URCs from Dümmen Orange after two weeks. In this trial after 39 days, Campanula had 100% rooting under indoor fog and mist, 98% in the light cart and dome, and 81% in the greenhouse.
Photos courtesy of University of Florida
Identify Your Return on Investment (ROI)
The ROI from indoor propagation is in comparison to the greenhouse. Therefore, optimize your greenhouse production space before building an indoor propagation facility, and document the level and causes of shrinkage. Greenhouses are likely to be more efficient for labor and plant movement than a growth room. Sunlight is cheap, but not free. Consider investments in LED lighting, shading, fog, cooling, and environmental controls in the greenhouse.
For most growers, indoor propagation is capital and labor-intensive and a small-scale option that is specialized for a subgroup of crops that have both high value and shrinkage. However, once you build a facility try to keep that space filled, even with easy-to-root plants, to spread out fixed costs.
Ballpark cost figures are $0.50 per tray per week for capital cost of LEDs plus electricity to run lights and cooling. We suggest doubling that figure to consider depreciation on other capital costs (including the insulated room, AC equipment, CO2 gas, etc.) and extra labor to care for plants and added touches in the production chain.
Improvements in labor, plant transport, and space use efficiency are likely in the future as growers have more experience with this emerging technology. For example, a shipping container may provide a low-cost air-conditioned grow room, but is likely to be inefficient for logistics. In your room design, can you enter from the sticking area at one end and connect the other end directly to the greenhouse? Can you fully automate irrigation with a combination of fog and subirrigation? Can you optimize vertical space between shelves for air movement, irrigation, and accessing plants? Can you propagate on the same rolling bench used in the greenhouse, or is it necessary to load and unload shipping carts?
The main ROI comes from:
- Reduced shrinkage (calculate the break-even number of plants that would successfully root per tray indoors compared with greenhouse propagation, to pay for the added costs). Three weeks at $1 per tray per week = $3 per tray. If each transplant has a net value of $0.50 per cutting, for example, you would need six (6 x $0.50 = $3) extra rooted cuttings per tray to break even.
- Reduced crop time in the greenhouse (if starting plants indoors cuts three weeks of crop time when moved to the greenhouse, that increases the number of turns you can provide in the greenhouse space). Calculate the additional break-even revenue you would need to generate from the greenhouse by producing more trays per season.
This is an exciting technology with other potential benefits, such as reduced pest pressure and increased quality and consistency indoors. The combination of research and grower ingenuity will advance indoor propagation systems.
Footnote: Thanks to George Grant, Megh Poudel, Gabriel Pelegrina, Joaquin Saavedra, Matias Yegros, and other students in the Fisher and Gómez labs for their assistance in these trials. The authors also appreciate advice and collaboration from Bob Hoffman at Shenandoah Growers, Marc Verdel at Battlefield Farms, Ty Strode and Robert Kizer at AgriStarts, and industry partners in the Floriculture Research Alliance.










