Managing Whiteflies in Poinsettias Using Biological Control Agents
Whiteflies are a common annual pest of ornamentals, and with the growth of insecticide-resistant species, retailer restrictions on specific insecticides, and increasing regulations on pesticide applicators, management of whiteflies is not getting any easier.
There are two main physically indistinguishable species of sweetpotato whiteflies we see in southern greenhouses, commonly referred to as MEAM1 (previously known as B-biotype) and MED (previously known as Q-biotype). How well a certain insecticide works against a particular whitefly population can vary greatly depending on the indistinguishable species present. Since the most reliable method to identify these species is to send off samples for molecular analysis, one strategy is to just assume you have a mixed population and use insecticides that have demonstrated good control against both cryptic species.
However, a whitefly management strategy relying solely on weekly insecticidal applications may be considered shortsighted due to the seemingly inevitable possibility of insecticide resistance. It also fails to consider an increasingly popular pest management strategy: augmentative biological control. This form of biological control utilizes commercially available predators or parasitic wasps that are released regularly (weekly or monthly) to manage a target pest.
When considering the use of biological control to manage pests in greenhouses, growers frequently voice two main concerns: 1) ornamentals have zero pest tolerance, and biological control cannot provide 100% control of pests, and 2) biological control is too expensive. These concerns have good merit, after all, previously published studies on using biological control to manage whiteflies on poinsettias commonly found costs to be much higher tha the cost of insecticide inputs. Additionally, entire ornamental plants are sold primarily for their aesthetic value. For this reason, a zero-pest tolerance is assumed – but any entomologist with a hand or head magnification lens knows that “zero” insects rarely means no insects. Below, we discuss our research that quantified the meaning of “zero” whiteflies at the retailer level and determine how well a biological control program can work when designed with some of the most recent research.

Adult parasitic wasp (Eretmocerus eremicus; left) and predatory mite (Amblyseius swirskii; middle). Parasitic wasps are often distributed on pupal cards hung near the plant canopy (right), and predatory mites are often distributed in slow-release sachets (not shown) or distributed through a modified blower on carrier material (seen on leaves in the image on the right). Koppert Biological Systems is one of many companies that produce and sell biological control agents; some others include Beneficial Insectary, Bioline AgroSciences, Applied Bio-nomics, and BioBest. Photos: Erfan Vafaie
How Many Is Zero?
It’s rarely possible to get 100% control of any particular insect pest; but perhaps populations can be low enough to be considered undetectable. To find out how many whiteflies is considered undetectable, we scouted poinsettias at several retailers in Texas over two separate years (2016 and 2018) and counted whitefly immatures and adults within 60 seconds per plant. Even though we only inspected poinsettias at retailers in Texas, poinsettias came from producers in Canada, California, and Texas. We found between 4 and 36 immature whiteflies in 2016 and between 25 and 73 immature whiteflies in 2018 per poinsettia on average, depending on the retailer. At a florist who inspects their poinsettias very closely and sells them at a premium ($50 per 6-inch poinsettia) we counted near 200 immature whiteflies on each of four poinsettias within 60 seconds. These whitefly densities are with current management practices, which in Texas are regular insecticide rotations. So “zero” is actually a quantifiable number and our goal is to maintain whitefly densities below this number in a biological control program. More details on this work and the results are published and available through the Journal of HortTechnology (Vafaie et al. 2020. “Whitefly abundance on rooted poinsettia cuttings”. HortTech, Volume 30: Issue 4).
Which Biological Control Species?
There are several species that are marketed as being effective at managing sweetpotato whiteflies, but there are two that have a history of data demonstrating effective and potentially economic control of whiteflies in greenhouse production: a parasitic wasp, Eretmocerus eremicus, and a predatory mite, Amblyseius swirskii. Growers are often left to decide whether to use multiple biological control species or double-down on a single species – so which is better? We conducted a series of trials with up to 12 poinsettias in isolated cages and consistently found that the combination of the predatory mite and parasitic wasp provided similar or superior suppression of whiteflies compared to either species alone. One potential explanation is that these two organisms feed on different life stages of whiteflies; the parasitic wasp prefers 2nd instar whitefly nymphs, whereas the predatory mite prefers whitefly eggs and 1st instar whitefly nymphs. Releasing too many wasps can result in the wasps competing with each other for the limited supply of 2nd instar whitefly nymphs, whereas adding predatory mites make available a previously invulnerable life stage (i.e., whitefly eggs). The results from this work are also being published and can be read in more detail (Vafaie et al. 2020. “A comparison of repetitive releases of single or multiple natural enemy species on the suppression of Bemisia tabaci infesting poinsettias”. Biological Control, Volume 151; Vafaie et al. 2020. “Adding a natural enemy to respond to pest immigration and delayed natural enemy releases in augmentative biological control”. Journal of Environmental Entomology, in press).
How Many Predators?
Using biological control as a curative treatment (i.e., applying when whitefly numbers are high) is cost prohibitive because the number to biological control agents needed to prevent aesthetic damage from the feed or presence of whitefly is great. Applications of insecticides that are considered compatible with the biological control agents are recommended when curative whitefly control is necessary. For augmentative biological control to be economically feasible, growers must initiate their program with low whitefly populations, use low density of biological control agents to suppress low-density populations of whiteflies, and spot spray with insecticides where populations start to rapidly increase (more on that in the next section). In the case of poinsettias, dipping unrooted cuttings in insecticidal soaps or biopesticides (also known as immersion treatments) before sticking can help start with low whitefly numbers. In a commercial-scale poinsettia biological control trial, we found that releasing between 1.8 to 2.6 E. eremicus pupae per m2 every week and between 27 to 44 A. swirskii per m2 every four weeks on average was economically comparable to insecticide inputs and within the recommended release density provided by commercial insectaries (e.g., Koppert Biological Systems recommends between 1.5 – 3 pupae/m2 for E. eremicus and between 25 – 50 mites/m2 for A. swirskii for preventative applications).
Monitoring Is Critical
In trials we conducted in commercial poinsettia production, we monitored a minimum of 50 poinsettias weekly out of 3,722 within a given greenhouse (about 1% of the crop). On average, it took us about 1 minute to inspect 20 leaves per plant when whitefly densities were low. We counted whiteflies of all life stages and tabulated the results over time.
Not only does systematic monitoring help determine when curative insecticide applications are needed to suppress whitefly numbers, it also helps inform at what whitefly densities we can expect the population to rapidly increase again in the future, allowing us to be proactive in our management. To draw these kinds of patterns, yellow sticky traps are insufficient. In reality, yellow sticky traps are not great at determining the density of insects on nearby crops, but rather, can serve as an early indicator of the presence of an insect. More elaborate monitoring of the individual plants and inspecting the undersides of leaves will produce a much more accurate idea of infestation levels in the crop.
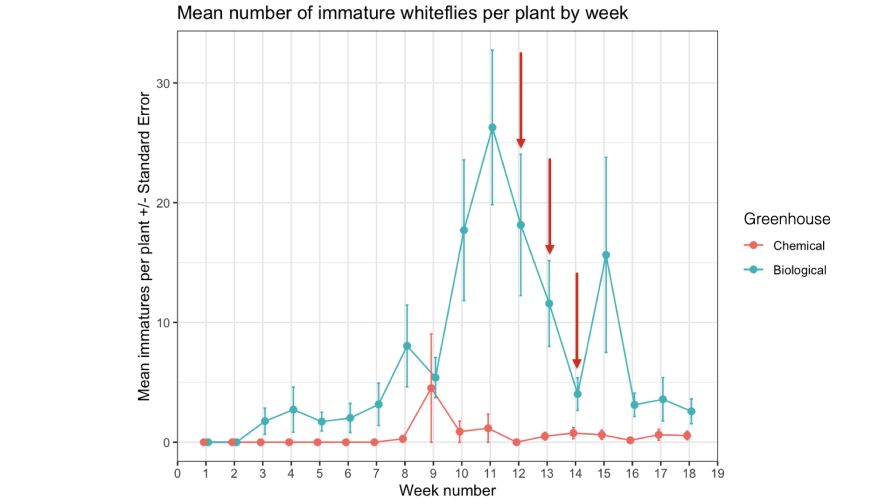
This chart shows the average number of immature whiteflies counted per poinsettia (and standard error; a measure of variance around the mean) in biological control and chemical control greenhouses over the duration of a commercial-scale poinsettia trial. Vertical red arrows denote when spot-spray insecticidal applications were made to reduce whitefly densities in the biological control greenhouse. The trends in whitefly populations over time inform when intervention with insecticidal applications are needed (i.e., rapid consecutive increase from weeks 9 to 11).
Can it Work?
We conducted a commercial-scale poinsettia experiment in 2019 with three participating growers. At each of the three commercial growers, we had two separate houses: one for biological control releases and another for conventional insecticide rotations. With weekly releases of E. eremicus and releases of A. swirskii every four weeks, we were ultimately able to provide similar whitefly densities in the biologically controlled greenhouses as the conventional insecticide greenhouses. Emphasis placed on ultimately, because at one of the commercial growers the density of whiteflies increased dramatically in the biologically controlled greenhouse, but a few (4 total) curative applications later, whitefly numbers were similar to the conventional insecticide greenhouses. Economics of biological control were comparable to conventional insecticides, costing anywhere between 0.6 to 3-fold the cost of the greenhouses management with conventional insecticide rotations. One of the reasons for this discrepancy in cost was related to the valuable information gathered through monitoring; growers had accurate and timely monitoring data available to them, and as a result, drastically reduced insecticidal applications in the conventional insecticide greenhouses due to low whitefly pressure – a lesson to be learned in the economic value of spraying insecticides informed by trends in monitoring data. By the end of the commercial trial, whitefly densities were well below average densities we found at retailers in 2016 and 2018. Details of our methodology, monitoring tactics, how economics were calculated, and other considerations are being published in the Journal of IPM (Vafaie et al. (2021). Using multiple natural enemies to manage whiteflies in commercial poinsettia production. Journal of IPM, in press).
Conclusions
Despite the belief that pest thresholds on ornamentals are “zero”, we found anywhere between 4 to 73 immature whiteflies on finished color poinsettias within 60 second counts, depending on the retailer and year. The two biological control that have demonstrated promise in replicated research experiments for management of whiteflies on poinsettias agents (E. eremicus and A. swirskii) work better together to suppress whiteflies compared to doubling the number of either biological control agent; so mixing the two is a better bang-for-your-buck than spending money on doubling an individual species. Management decisions based on monitoring data can potentially drastically reduce insecticidal applications when whitefly pressure is low, proving to be one of the more cost-effective strategies. Lastly, we found that a whitefly management program based on the release of the parasitic wasp (E. eremicus) and predatory mite (A. swirskii), with curative insecticidal applications applied based on monitoring data, can provide similar whitefly densities and be economically competitive to insecticide-based management programs.









